Vaginitis Diagnostics Market : Testing Type (Nucleic Acid Amplification Assays (NAATs), DNA Probes, Point-of-Care Testing (POCT), Clinical Tests and Others); Disease Type (Infectious Disease Type and Non-infectious Disease Type); End-user (Hospitals, Diagnostic Laboratories, Speciality Clinics and Home care settings)
Market Outlook
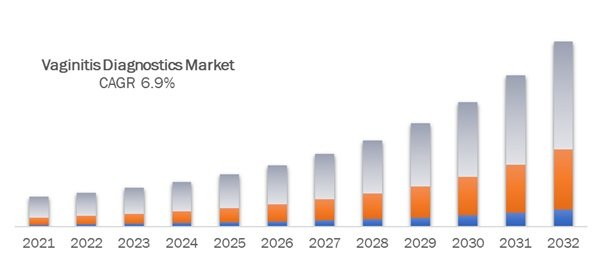
The global vaginitis diagnostics market is projected to witness substantial growth, driven by an increasing prevalence of vaginal infections and advancements in diagnostic technologies. The market is expected to reach over US$ 3.4 billion by 2024, with a compound annual growth rate (CAGR) of approximately 6.9% during the forecast period from 2025 to 2032.
Major drivers here are the increasing incidence of bacterial vaginosis, vulvovaginal candidiasis, and trichomoniasis, which demand effective diagnostic solutions. Technological innovation in the form of nucleic acid amplification tests (NAATs) and point-of-care tests is enhancing diagnostic accuracy as well as efficiency, thus driving the market. Greater awareness regarding the health issues of women is also convincing more women towards early diagnosis as well as treatments. However, the market is constrained by high costs of newer diagnostic tests as well as poor awareness in developing nations.
Opportunities lie in the expansion of telemedicine services and the development of innovative point-of-care testing solutions that can improve accessibility and convenience for patients. Overall, the vaginitis diagnostics market is positioned for robust growth, with stakeholders focusing on technological advancements and strategic collaborations to enhance their market presence and address the evolving needs of women’s health.
Drivers
Increasing Incidence of Vaginitis
The rising cases of vaginitis, BV, and VVC point specifically to the pressing need for more advanced diagnostic capabilities. An estimated 30% of U.S. women alone have bacterial vaginosis, the number one condition affecting the vagina. These statistics translate to more than just numbers—they translate to actual women living with the irritation, broken daily routines, and preterm birth complications or complications from pelvic inflammatory disease when the condition is left unattended.
The physical and emotional burden of vaginitis underscores the need for precise, dependable, and quick diagnostic procedures to facilitate timely care and intervention. Through enhanced diagnosis, healthcare professionals can avert misdiagnosis, avoid recurrent infections, and provide improved patient experiences, which in turn will drive the diagnostics market.
Technological Advancements in Diagnostic Tools
Innovations in diagnostic technologies are revolutionizing the detection and management of vaginitis. Tools like nucleic acid amplification tests (NAATs) and syndromic testing panels deliver faster and more accurate results compared to traditional methods. For example, the multiplex PCR (Polymerase Chain Reaction) test allows clinicians to simultaneously identify multiple pathogens, such as Candida, Gardnerella vaginalis, and Trichomonas vaginalis. Imagine a woman who has struggled with recurrent infections finally receiving a precise diagnosis in a single visit—this level of efficiency reduces anxiety and ensures prompt, targeted treatment. These advancements empower healthcare professionals to make informed decisions quickly, improving patient outcomes and driving significant growth in the diagnostics market.
Growing Awareness and Education
Awareness and education play a vital role in addressing vaginitis. Increased public health campaigns and educational initiatives encourage women to recognize symptoms and seek medical help early. When women are informed about the importance of accurate diagnosis and treatment, they are more likely to visit healthcare providers instead of relying on over-the-counter solutions.
Restraints
Lack of Awareness in Developing Regions
In many developing regions, the condition of vaginitis is also surrounded with social taboo, limited healthcare literacy, as well as misinformation. Rural women also experience the symptoms of itchiness, abnormal discharge, or irritation but tend to disregard them due to limited awareness or social stigma. Talking about intimate health is also taboo, which makes professional intervention difficult for the women. In these regions, the healthcare infrastructure is also weak with limited diagnostic centers or skilled healthcare providers who can perform timely diagnosis.
In addition, outreach for women’s reproductive health is either non-existent or sporadic, and most are unaware of how vaginitis can lead to complications like pelvic inflammatory disease or sterility. Such a cycle of underdiagnoses and lack of awareness not only adds to the health of the individual but also suppresses market growth for diagnostic devices and solutions in these regions.
Self-Diagnosis and Treatment Practices
In the digital age, an increasing number of women turn to online resources for health information. Platforms like blogs, social media, and health forums provide anecdotal advice that can seem reassuring. However, this practice of self-diagnosing vaginitis based on symptoms alone is fraught with risks. Vaginal infections can be caused by bacteria, yeast, or parasites, and distinguishing between these types requires professional diagnostic tests. Misdiagnosis often leads to incorrect or unnecessary treatments, such as the overuse of antifungal creams when a bacterial infection is present.
Moreover, many women opt for home remedies or over-the-counter products based on incomplete information. While these treatments may offer temporary relief, they often mask symptoms rather than addressing the underlying cause. This not only prolongs discomfort but may also lead to chronic infections. The reliance on self-diagnosis diminishes the perceived need for professional diagnostic services, restraining market growth and delaying appropriate medical care.
Opportunities
Expansion of Telemedicine Services: Enhancing Access to Vaginitis Diagnostics
The rise of telemedicine solutions is changing the method through which people access healthcare, notably the diagnosis of vaginitis. It is more than convenience; telemedicine bridges the gap for patients who live in rural, remote, or underserved areas with limited traditional healthcare facilities. Virtual visits allow patients to view healthcare providers from the comforts of their homes, facilitating timely diagnosis as well as the required treatments for conditions like bacterial vaginosis, yeast infections, as well as other conditions involving the vagina.
Telemedicine also alleviates mobility, transportation, and time-based impediments, bringing healthcare to working professionals, caregivers, as well as the economically underprivileged. Telemedicine also frees patients from the anxiety of loss of autonomy as well as confidentiality, which would have otherwise deterred them from accessing the required attention. Telemedicine platforms can also include educative materials, through which patients can be aware of their conditions as well as when they can seek the attention of healthcare providers. By extending telemedicine coverage, healthcare organizations can develop a more patient-centric, more productive, as well as more inclusive system for the management of vaginitis.
Development of Point-of-Care Testing (POCT): Bringing Diagnostics Closer to Patients
The evolution of point-of-care testing (POCT) offers a transformative opportunity for diagnosing vaginitis in real-time, whether at home, in primary care clinics, or community health settings. POCT solutions provide rapid results without the need for sophisticated laboratory infrastructure, reducing the waiting time between symptom onset, diagnosis, and treatment. This immediate feedback not only alleviates patient anxiety but also enhances adherence to recommended care plans.
For patients, particularly those who experience recurring infections or face challenges accessing traditional clinics, POCT provides a sense of control and reassurance. The simplicity and speed of these tests can improve early detection and intervention, helping to prevent complications and discomfort. Additionally, POCT encourages proactive healthcare behaviors, empowering individuals to monitor their health more regularly. As healthcare systems prioritize patient-centered approaches, developing reliable, accurate, and user-friendly POCT devices represents a pivotal step in enhancing diagnostic accessibility and improving overall women’s health outcomes.
Both telemedicine and POCT innovations signify a broader movement toward democratizing healthcare, ensuring that high-quality diagnostics and care are available to everyone, regardless of location or socioeconomic status.
Competitive Landscape
| Company Name | Products/Brands |
| Laboratory Corporation of America | Various diagnostic services including vaginal infection tests |
| Cepheid (Danaher Corporation) | Xpert BV |
| Abbott Laboratories | Alere |
| QIAGEN | QIAsymphony |
| Roche Diagnostics | cobas 4800 System |
| Hologic | APTIMA |
| Quidel Corporation | Sofia |
| Quest Diagnostics | Quest Vaginitis Testing |
| Atrida | BVBlue |
| Becton, Dickinson and Company | BD Max |
| bioMérieux | Vidas |
| Sekisui Diagnostics | Vaginal Infections Test |
| Binx Health | Binx Rapid Test |
| Thermo Fisher Scientific | Ion Torrent |
| PHASE Scientific | PHASE Bacterial Vaginosis Test |
Recent Developments
- In October 2024, Quest Diagnostics is introducing a new service through which patients can self-collect specimens for routine genital tract infection (GTI) testing at its national patient service center network. It is the first-of-its-kind service, which is being put in place to provide patients with more discreet and convenient diagnosis and treatment for conditions such as vaginitis, chlamydia, gonorrhea, trichomoniasis, and Mycoplasma genitalium. It is free at any of the 2,000 patient service centers with a physician’s order or through the consumer-initiated test program at questhealth.com.
- In January 2024, Cepheid has received FDA clearance for its Xpert® Xpress MVP, a multiplex vaginal panel for Bacterial Vaginosis, Vulvovaginal Candidiasis, and Trichomoniasis. The test, which runs on Cepheid’s GeneXpert Xpress instruments, aims to provide targeted treatments for vaginitis, a common condition affecting 10 million healthcare visits annually.
Market Segmentation
| Vaginitis Diagnostics Market | |
| Testing Type | o Nucleic Acid Amplification Assays (NAATs)
o DNA Probes o Point-of-Care Testing (POCT) o Clinical Tests o Others (Whiff test etc.) |
| Disease Type | o Infectious types
§ Bacterial Vaginosis (BV) § Vulvovaginal Candidiasis (Yeast Infection) § Trichomoniasis o Non-infectious types § Atrophic Vaginitis § Irritant Vaginitis § Allergic Vaginitis § Inflammatory Vaginitis |
| End-user | Hospitals
Diagnostic Laboratories Specialty Clinics Home care Settings |
| Distribution Channel | o Veterinary Hospitals/Clinics
o Retail Pharmacies o Online Platforms o Veterinary Practitioners |
| Region | o North America
o Europe o Asia Pacific o Latin America o Middle East & Africa |
Frequently Asked Questions for Vaginitis Diagnostics Market
What is the market size of the global Vaginitis Diagnostics industry?
The market size for the global Vaginitis Diagnostics market is US$ 6.3 Billion in 2032.
What is the compound annual growth rate (CAGR) of the global Vaginitis Diagnostics market?
The market is expected to grow at a compound annual growth rate (CAGR) of 6.9% from 2025 to 2032.
What factors are driving the growth of this market?
Increasing adoption of point-of-care testing solutions for rapid diagnosis.
Which companies are the key players in the Vaginitis Diagnostics market?
Leading companies operating in the Vaginitis Diagnostics market are Abbott Laboratories, BD (Becton, Dickinson and Company), Thermo Fisher Scientific, Quest Diagnostics Incorporated and Other prominent players.
Which region holds the largest market share?
North America region hold largest market share in vaginitis diagnostics market
ToC
|

