View TOC
Global Contract Research Organization (CRO) Market Report, By Type of Services (Clinical Research Services, Preclinical Services, Laboratory Services and Consulting Services); By Therapeutic Area (Oncology, Cardiovascular Diseases, Neurology, Infectious Diseases, Metabolic Disorders, Rare and Orphan Diseases and Other Therapeutic Areas); Business Model (Full-Service CROs, Functional Service Providers (FSPs) and Hybrid Models); End-user (Pharmaceutical Companies, Biotechnology Companies, Academic Institutions & Research Centers, Government Organizations and Others)
Market Outlook
The global Contract Research organisations market is experiencing significant growth trend, driven by Pharmaceutical and biotech companies are investing heavily in R&D, leading to increased demand for CRO services in drug discovery, preclinical studies, and clinical trials. The global CROs market is Valued at approximately US$ 85.1 Billion in 2024, the market is projected to reach US$ 207.7 Billion by 2032, with a compound annual growth rate (CAGR) of 10.8% during the forecast period from 2025 to 2032.

Growing R&D Expenditure Driving CRO Market Growth
Pharmaceutical and biotechnology firms are increasing their R&D investments, creating greater demand for Contract Research Organizations (CROs) to support drug discovery, preclinical research, and clinical trials. For Instance, Charles River Laboratories is a leading global CRO that offers preclinical and early-stage drug development services. Charles River Laboratories reported that it has agreed to acquire Cognate BioServices, Inc. for approximately US$875 million in cash. The acquisition significantly expands Charles River’s presence in the fast-growing cell and gene therapy (CGT) market, complementing its contract development and manufacturing organization (CDMO) capabilities. Charles River is already a leader in preclinical CRO. With Cognate’s acquisition, it can now offer end-to-end services to CGT companies, from early discovery through clinical and commercial manufacturing.
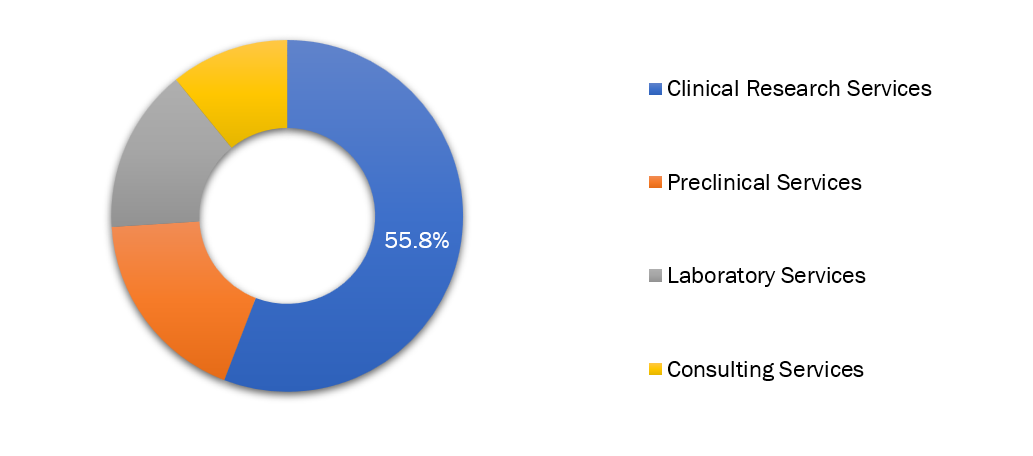
The clinical research services segment leads the global CRO market.
Clinical research services is the leading segment in the worldwide Contract Research Organization (CRO) market, driven by increasing complexity in clinical trials and high outsourcing from pharmaceutical and biotechnology companies.
Increasing personalized medicine, rare disease research, and oncology trials have further fueled demand for specialized clinical research services. Furthermore, advancements in AI-optimized trial design, decentralized trials, and digital health technology have improved the efficiency of CROs, attracting more sponsors. For instance, One of such advancements in this space is Lindus Health’s launch of an “All-In-One CRO for Digital Therapeutics” solution. The end-to-end solution combines technology, site services, and CRO capabilities to maximize clinical trials for digital therapeutics (DTx) and digital health interventions. Lindus Health has already conducted 17 DTx studies across 12 disease indications in the US, UK, and EU, with over 3,000 patients enrolled.
Additionally, elite CROs such as Syneos Health, Parexel, and Labcorp Drug Development dominate this segment with their end-to-end clinical trial services for various therapeutic areas. Amidst rising investments in R&D activities across the globe and escalating regulatory requirements, the clinical research services segment continues to be the key revenue generator in the CRO industry.
Regional Analysis
The North American Contract Research Organization (CRO) market is set to witness significant growth from 2021 to 2032, North America is the most significant contributor to the Contract Research Organization (CRO) market due to the fact that it has a well-developed pharmaceutical and biotechnology industry, high R&D investments, and a strong regulatory framework led by the FDA. The presence of major CRO providers like IQVIA, Parexel, Labcorp Drug Development, and Charles River Laboratories in the region further solidifies the region’s dominance. North America has the added advantage of high adoption of technologies like AI-driven research and decentralized clinical trials that enhance efficiency and attract clients globally.
On the other hand, the most rapidly developing market for CROs is the Asia Pacific region, driven by cost-effective clinical trial services, a large and diverse patient population, and increasing clinical trial activities in countries like China, India, and South Korea. Increasing pharmaceutical and biotech sectors, coupled with increasing foreign investments, are also boosting market growth. Accordingly, global CROs are actively expanding their businesses in Asia Pacific, leveraging lower operational costs and faster patient recruitment to accelerate drug development.
Contract Research Organization (CRO) Market Key Industry Updates
On January 13, 2025, Caidya, a mid-sized global CRO, announced that it has secured a US$165 million strategic growth investment from US-based healthcare investment firm Rubicon Founders. The investment will be utilized to accelerate Caidya’s future organic growth and strategic acquisitions.
In April 2024, Parexel and Palantir Technologies Inc. announced a multi-year strategic partnership to leverage AI for the enhancement and acceleration of the delivery of safe and effective clinical trials to the world’s biopharmaceutical customers. Under the partnership, Parexel will leverage Palantir’s Foundry and Artificial Intelligence Platform (AIP) to advance its clinical data platform, with the objective of advancing clinical trial efficiency without sacrificing the highest levels of safety and regulatory stringency.
In August 2024, Silo Pharma, Inc. entered into an agreement with WuXi AppTec (Hong Kong) Limited, a leading global contract research organization (CRO), for the preclinical small animal study of SPU-16, a central nervous system (CNS) homing peptide for multiple sclerosis (MS). Silo Pharma is developing the SPU-16 liposomal homing peptide pursuant to a commercial evaluation license and option agreement with the University of Maryland, Baltimore (UMB).
Contract Research Organization (CRO) Industry Competition Landscape
| IQVIA | SGS Life Sciences |
| Labcorp Drug Development | Piramal Pharma Solutions |
| ICON plc | Jubilant Biosys |
| Parexel International | GVK BIO (Aragen Life Sciences) |
| Syneos Health | Celerion |
| Charles River Laboratories | Frontage Laboratories |
| PPD (Thermo Fisher Scientific) | Eurofins Scientific |
| Medpace | Clinipace |
| PRA Health Sciences (Acquired by ICON plc) | Veristat |
| WuXi AppTec | Syngene International |
Global Contract Research Organization (CRO) Market Segmentation
Segmentation by Type of Services
- Clinical Research Services
- Phase I Trials
- Phase II Trials
- Phase III Trials
- Phase IV Trials
- Clinical Monitoring
- Data Management
- Regulatory Affairs
- Preclinical Services
- Toxicology
- Acute Toxicity Testing
- Chronic Toxicity Studies
- Pharmacology
- Pharmacokinetics and Pharmacodynamics
- Dose-Response Studies
- Laboratory Services
- Bioanalytical Services
- Sample Analysis
- Clinical Biomarker Testing
- Central Laboratory Services
- Genomic Testing Services
- Consulting Services
- Regulatory Consulting
- Market Access Consulting
- Product Development Consulting
Segmentation by Therapeutic Area
- Oncology
- Cardiovascular Diseases
- Neurology
- Infectious Diseases
- Metabolic Disorders
- Rare and Orphan Diseases
- Other Therapeutic Areas
Segmentation by Business Model
- Full-Service CROs
- End-to-end clinical research services
- Global vs. local CROs
- Functional Service Providers (FSPs)
- Specialized Services
- Flexible outsourcing models
- Hybrid Models
- Full-service and functional services
- Customizable Solutions
Segmentation by End-user
- Pharmaceutical Companies
- Biotechnology Companies
- Academic Institutions & Research Centers
- Government Organizations
- Others
Segmentation by Region
- North America
- S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Denmark
- Rest of Europe
- Asia Pacific
- Japan
- China
- India
- Australia & New Zealand
- South Korea
- Singapore
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Colombia
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of Middle East & Africa
Table Of Content
| 1. Executive Summary |
| 1.1. Definition |
| 1.2. Research Scope |
| 1.3. Key Findings by Major Segments |
| 2. Global Contract Research Organization (CRO) Market Overview |
| 2.1. Contract Research Organization (CRO) Market Dynamics |
| 2.1.1. Drivers |
| 2.1.2. Opportunities |
| 2.1.3. Restraints |
| 2.1.4. Challenges |
| 2.2. List of Major CROs, By Key Countries |
| 2.3. Regulatory Scenario, By Region |
| 2.4. Government Incentives Available for Clinical Trials in India |
| 2.5. Key Industry Updates |
| 3. Global Contract Research Organization (CRO) Market Outlook and Future Prospects, 2021-2032 |
| 3.1. Global Contract Research Organization (CRO) Market Analysis, 2021-2023 |
| 3.2. Global Contract Research Organization (CRO) Market Opportunity and Forecast, 2025-2032 |
| 3.3. Global Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Type of Services, 2021-2032 |
| 3.3.1. Global Contract Research Organization (CRO) Market Analysis by Type of Services: Introduction |
| 3.3.2. Market Trend, Analysis and Forecast, By Type of Services, 2021-2032 |
| 3.3.2.1. Clinical Research Services |
| Phase I Trials |
| Phase II Trials |
| Phase III Trials |
| Phase IV Trials |
| Clinical Monitoring |
| Data Management |
| Regulatory Affairs |
| 3.3.2.2. Preclinical Services |
| Toxicology |
| Acute Toxicity Testing |
| Chronic Toxicity Studies |
| Pharmacology |
| Pharmacokinetics and Pharmacodynamics |
| Dose-Response Studies |
| 3.3.2.3. Laboratory Services |
| Bioanalytical Services |
| Sample Analysis |
| Clinical Biomarker Testing |
| Central Laboratory Services |
| Genomic Testing Services |
| 3.3.2.4. Consulting Services |
| Regulatory Consulting |
| Market Access Consulting |
| Product Development Consulting |
| 3.4. Global Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Therapeutic Area, 2021-2032 |
| 3.4.1. Global Contract Research Organization (CRO) Market Analysis by Therapeutic Area: Introduction |
| 3.4.2. Market Trend, Analysis and Forecast, By Therapeutic Area, 2021-2032 |
| 3.4.2.1. Oncology |
| 3.4.2.2. Cardiovascular Diseases |
| 3.4.2.3. Neurology |
| 3.4.2.4. Infectious Diseases |
| 3.4.2.5. Metabolic Disorders |
| 3.4.2.6. Rare and Orphan Diseases |
| 3.4.2.7. Others |
| 3.5. Global Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Business Model, 2021-2032 |
| 3.5.1. Global Contract Research Organization (CRO) Market Analysis by Business Model: Introduction |
| 3.5.2. Market Trend, Analysis and Forecast, By Business Model, 2021-2032 |
| 3.5.2.1. Full-Service CROs |
| 3.5.2.2. Functional Service Providers (FSPs) |
| 3.5.2.3. Hybrid Models |
| 3.8. Global Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By End-user, 2021-2032 |
| 3.8.1. Global Contract Research Organization (CRO) Market Analysis by End-user: Introduction |
| 3.8.2. Market Trend, Analysis and Forecast, By End-user, 2021-2032 |
| 3.8.2.1. Pharmaceutical Companies |
| 3.8.2.2. Biotechnology Companies |
| 3.8.2.3. Academic Institutions & Research Centers |
| 3.8.2.4. Government Organizations |
| 3.8.2.5. Others |
| 3.9. Global Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Region, 2021-2032 |
| 3.9.1. Global Contract Research Organization (CRO) Market Analysis by Region : Introduction |
| 3.9.2. Market Trend, Analysis and Forecast, By Region , 2021-2032 |
| 3.9.2.1. North America |
| 3.9.2.2. Europe |
| 3.9.2.4. APAC |
| 3.9.2.5. Latin America |
| 3.9.2.6. Middle East & Africa |
| 4. North America Contract Research Organization (CRO) Market Outlook and Future Prospects, 2021-2032 |
| 4.1. North America Contract Research Organization (CRO) Market Analysis, 2021-2023 |
| 4.2. North America Contract Research Organization (CRO) Market Opportunity and Forecast, 2025-2032 |
| 4.3. North America Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Type of Services, 2021-2032 |
| 4.3.1. Clinical Research Services |
| Phase I Trials |
| Phase II Trials |
| Phase III Trials |
| Phase IV Trials |
| Clinical Monitoring |
| Data Management |
| Regulatory Affairs |
| 4.3.2. Preclinical Services |
| Toxicology |
| Acute Toxicity Testing |
| Chronic Toxicity Studies |
| Pharmacology |
| Pharmacokinetics and Pharmacodynamics |
| Dose-Response Studies |
| 4.3.3. Laboratory Services |
| Bioanalytical Services |
| Sample Analysis |
| Clinical Biomarker Testing |
| Central Laboratory Services |
| Genomic Testing Services |
| 4.3.4. Consulting Services |
| Regulatory Consulting |
| Market Access Consulting |
| Product Development Consulting |
| 4.4. North America Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Therapeutic Area, 2021-2032 |
| 4.4.1. Oncology |
| 4.4.2. Cardiovascular Diseases |
| 4.4.3. Neurology |
| 4.4.4. Infectious Diseases |
| 4.4.5. Metabolic Disorders |
| 4.4.6. Rare and Orphan Diseases |
| 4.4.7. Other Therapeutic Areas |
| 4.5. North America Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Business Model, 2021-2032 |
| 4.5.1. Full-Service CROs |
| End-to-end clinical research services |
| Global vs. local CROs |
| 4.5.2. Functional Service Providers (FSPs) |
| Specialized Services |
| Flexible outsourcing models |
| 4.5.3. Hybrid Models |
| Full-service and functional services |
| Customizable Solutions |
| 4.6. North America Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By End-user, 2021-2032 |
| 4.6.1. Pharmaceutical Companies |
| 4.6.2. Biotechnology Companies |
| 4.6.3. Academic Institutions & Research Centers |
| 4.6.4. Government Organizations |
| 4.6.5. Others |
| 4.7. North America Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Country, 2021-2032 |
| 4.7.1. U.S. |
| 4.7.2. Canada |
| 5. Europe Contract Research Organization (CRO) Market Outlook and Future Prospects, 2021-2032 |
| 5.1. Europe Contract Research Organization (CRO) Market Analysis, 2021-2023 |
| 5.2. Europe Contract Research Organization (CRO) Market Opportunity and Forecast, 2025-2032 |
| 5.3. Europe Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Type of Services, 2021-2032 |
| 5.3.1. Clinical Research Services |
| Phase I Trials |
| Phase II Trials |
| Phase III Trials |
| Phase IV Trials |
| Clinical Monitoring |
| Data Management |
| Regulatory Affairs |
| 5.3.2. Preclinical Services |
| Toxicology |
| Acute Toxicity Testing |
| Chronic Toxicity Studies |
| Pharmacology |
| Pharmacokinetics and Pharmacodynamics |
| Dose-Response Studies |
| 5.3.3. Laboratory Services |
| Bioanalytical Services |
| Sample Analysis |
| Clinical Biomarker Testing |
| Central Laboratory Services |
| Genomic Testing Services |
| 5.3.5. Consulting Services |
| Regulatory Consulting |
| Market Access Consulting |
| Product Development Consulting |
| 5.4. Europe Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Therapeutic Area, 2021-2032 |
| 5.5.1. Oncology |
| 5.5.2. Cardiovascular Diseases |
| 5.5.3. Neurology |
| 5.5.5. Infectious Diseases |
| 5.5.5. Metabolic Disorders |
| 5.5.6. Rare and Orphan Diseases |
| 5.5.7. Other Therapeutic Areas |
| 5.5. Europe Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Business Model, 2021-2032 |
| 5.5.1. Full-Service CROs |
| End-to-end clinical research services |
| Global vs. local CROs |
| 5.5.2. Functional Service Providers (FSPs) |
| Specialized Services |
| Flexible outsourcing models |
| 5.5.3. Hybrid Models |
| Full-service and functional services |
| Customizable Solutions |
| 5.6. Europe Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By End-user, 2021-2032 |
| 5.6.1. Pharmaceutical Companies |
| 5.6.2. Biotechnology Companies |
| 5.6.3. Academic Institutions & Research Centers |
| 5.6.5. Government Organizations |
| 5.6.5. Others |
| 5.7. Europe Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Country, 2021-2032 |
| 5.7.1. Germany |
| 5.7.2. UK |
| 5.7.3. France |
| 5.7.5. Spain |
| 5.7.5. Italy |
| 5.7.6. Denmark |
| 5.7.7. Rest of Europe |
| 6. Asia Pacific Contract Research Organization (CRO) Market Outlook and Future Prospects, 2021-2032 |
| 6.1. Asia Pacific Contract Research Organization (CRO) Market Analysis, 2021-2023 |
| 6.2. Asia Pacific Contract Research Organization (CRO) Market Opportunity and Forecast, 2025-2032 |
| 6.3. Asia Pacific Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Type of Services, 2021-2032 |
| 6.3.1. Clinical Research Services |
| Phase I Trials |
| Phase II Trials |
| Phase III Trials |
| Phase IV Trials |
| Clinical Monitoring |
| Data Management |
| Regulatory Affairs |
| 6.3.2. Preclinical Services |
| Toxicology |
| Acute Toxicity Testing |
| Chronic Toxicity Studies |
| Pharmacology |
| Pharmacokinetics and Pharmacodynamics |
| Dose-Response Studies |
| 6.3.3. Laboratory Services |
| Bioanalytical Services |
| Sample Analysis |
| Clinical Biomarker Testing |
| Central Laboratory Services |
| Genomic Testing Services |
| 6.3.6. Consulting Services |
| Regulatory Consulting |
| Market Access Consulting |
| Product Development Consulting |
| 6.6. Asia Pacific Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Therapeutic Area, 2021-2032 |
| 6.6.1. Oncology |
| 6.6.2. Cardiovascular Diseases |
| 6.6.3. Neurology |
| 6.6.6. Infectious Diseases |
| 6.6.5. Metabolic Disorders |
| 6.6.6. Rare and Orphan Diseases |
| 6.6.7. Other Therapeutic Areas |
| 6.5. Asia Pacific Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Business Model, 2021-2032 |
| 6.5.1. Full-Service CROs |
| End-to-end clinical research services |
| Global vs. local CROs |
| 6.5.2. Functional Service Providers (FSPs) |
| Specialized Services |
| Flexible outsourcing models |
| 6.5.3. Hybrid Models |
| Full-service and functional services |
| Customizable Solutions |
| 6.6. Asia Pacific Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By End-user, 2021-2032 |
| 6.6.1. Pharmaceutical Companies |
| 6.6.2. Biotechnology Companies |
| 6.6.3. Academic Institutions & Research Centers |
| 6.6.6. Government Organizations |
| 6.6.5. Others |
| 6.7. Asia Pacific Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Country, 2021-2032 |
| 6.7.1. China |
| 6.7.2. Japan |
| 6.7.3. India |
| 6.7.4. Australia & New Zealand |
| 6.7.5. South Korea |
| 6.7.6. Singapore |
| 6.7.7. Rest of Asia Pacific |
| 7. Latin America Contract Research Organization (CRO) Market Outlook and Future Prospects, 2021-2032 |
| 7.1. Latin America Contract Research Organization (CRO) Market Analysis, 2021-2023 |
| 7.2. Latin America Contract Research Organization (CRO) Market Opportunity and Forecast, 2025-2032 |
| 7.3. Latin America Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Type of Services, 2021-2032 |
| 7.3.1. Clinical Research Services |
| Phase I Trials |
| Phase II Trials |
| Phase III Trials |
| Phase IV Trials |
| Clinical Monitoring |
| Data Management |
| Regulatory Affairs |
| 7.3.2. Preclinical Services |
| Toxicology |
| Acute Toxicity Testing |
| Chronic Toxicity Studies |
| Pharmacology |
| Pharmacokinetics and Pharmacodynamics |
| Dose-Response Studies |
| 7.3.3. Laboratory Services |
| Bioanalytical Services |
| Sample Analysis |
| Clinical Biomarker Testing |
| Central Laboratory Services |
| Genomic Testing Services |
| 7.3.7. Consulting Services |
| Regulatory Consulting |
| Market Access Consulting |
| Product Development Consulting |
| 7.4. Latin America Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Therapeutic Area, 2021-2032 |
| 7.4.1. Oncology |
| 7.4.2. Cardiovascular Diseases |
| 7.4.3. Neurology |
| 7.4.7. Infectious Diseases |
| 7.4.5. Metabolic Disorders |
| 7.4.6. Rare and Orphan Diseases |
| 7.4.7. Other Therapeutic Areas |
| 7.5. Latin America Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Business Model, 2021-2032 |
| 7.5.1. Full-Service CROs |
| End-to-end clinical research services |
| Global vs. local CROs |
| 7.5.2. Functional Service Providers (FSPs) |
| Specialized Services |
| Flexible outsourcing models |
| 7.5.3. Hybrid Models |
| Full-service and functional services |
| Customizable Solutions |
| 7.6. Latin America Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By End-user, 2021-2032 |
| 7.6.1. Pharmaceutical Companies |
| 7.6.2. Biotechnology Companies |
| 7.6.3. Academic Institutions & Research Centers |
| 7.6.7. Government Organizations |
| 7.6.5. Others |
| 7.7. Latin America Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Country, 2021-2032 |
| 7.7.1. Brazil |
| 7.7.2. Mexico |
| 7.7.3. Colombia |
| 7.7.4. Rest of Latin America |
| 8. Middle East & Africa Contract Research Organization (CRO) Market Outlook and Future Prospects, 2021-2032 |
| 8.1. Middle East & Africa Contract Research Organization (CRO) Market Analysis, 2021-2023 |
| 8.2. Middle East & Africa Contract Research Organization (CRO) Market Opportunity and Forecast, 2025-2032 |
| 8.3. Middle East & Africa Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Type of Services, 2021-2032 |
| 8.3.1. Clinical Research Services |
| Phase I Trials |
| Phase II Trials |
| Phase III Trials |
| Phase IV Trials |
| Clinical Monitoring |
| Data Management |
| Regulatory Affairs |
| 8.3.2. Preclinical Services |
| Toxicology |
| Acute Toxicity Testing |
| Chronic Toxicity Studies |
| Pharmacology |
| Pharmacokinetics and Pharmacodynamics |
| Dose-Response Studies |
| 8.3.3. Laboratory Services |
| Bioanalytical Services |
| Sample Analysis |
| Clinical Biomarker Testing |
| Central Laboratory Services |
| Genomic Testing Services |
| 8.3.8. Consulting Services |
| Regulatory Consulting |
| Market Access Consulting |
| Product Development Consulting |
| 8.4. Middle East & Africa Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Therapeutic Area, 2021-2032 |
| 8.4.1. Oncology |
| 8.4.2. Cardiovascular Diseases |
| 8.4.3. Neurology |
| 8.4.8. Infectious Diseases |
| 8.4.5. Metabolic Disorders |
| 8.4.6. Rare and Orphan Diseases |
| 8.4.7. Other Therapeutic Areas |
| 8.5. Middle East & Africa Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Business Model, 2021-2032 |
| 8.5.1. Full-Service CROs |
| End-to-end clinical research services |
| Global vs. local CROs |
| 8.5.2. Functional Service Providers (FSPs) |
| Specialized Services |
| Flexible outsourcing models |
| 8.5.3. Hybrid Models |
| Full-service and functional services |
| Customizable Solutions |
| 8.6. Middle East & Africa Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By End-user, 2021-2032 |
| 8.6.1. Pharmaceutical Companies |
| 8.6.2. Biotechnology Companies |
| 8.6.3. Academic Institutions & Research Centers |
| 8.6.8. Government Organizations |
| 8.6.5. Others |
| 8.7. Middle East & Africa Contract Research Organization (CRO) Market Analysis, Opportunity and Forecast, By Country, 2021-2032 |
| 8.7.1. GCC Countries |
| 8.7.2. South Africa |
| 8.7.3. Rest of Middle East & Africa |
| 9. Global Contract Research Organization (CRO) Market Competitive Landscape, Market Share Analysis, and Company Profiles |
| 9.1. Contract Research Organization (CRO) Market Share Analysis, By Company (2024) |
| 9.2. Company Profiles |
| 1. IQVIA |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 2. Labcorp |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 3. ICON plc |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 4. Parexel International |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 5. Syneos Health |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 6. Charles River Laboratories |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 7 PPD (Thermo Fisher Scientific) |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 8. Medpace |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 9. PRA Health Sciences |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 10. WuXi AppTec |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 11. SGS Life Sciences |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 12. Piramal Pharma Solutions |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 13. Jubilant Biosys |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 14. GVK BIO (Aragen Life Sciences) |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 15. Celerion |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 16. Frontage Laboratories |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 17. Eurofins Scientific |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 18. Clinipace |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 19. Veristat |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 20. Syngene International |
| Company Overview |
| Financial Highlights |
| Product Portfolio |
| SWOT Analysis |
| Key Strategies and Developments |
| 9.3. Competitive Comparison Matrix |
| 10. Research Methodology |
| 11. Conclusion and Recommedations |
Frequently Asked Questions for Contract Research Organization (CRO) Market
What is the market size of the global Contract Research Organization (CRO) industry?
The market size for the global Contract Research Organization (CRO) market is US$ 85.1 Billion in 2024.
What is the compound annual growth rate (CAGR) of the global Contract Research Organization (CRO) market?
The market is expected to grow at a compound annual growth rate (CAGR) of 10.8% from 2025 to 2032.
What factors are driving the growth of this market?
The growing need for specialized expertise in oncology, rare diseases, and personalized medicine is boosting the demand for CROs market
Which companies are the key players in the Contract Research Organization (CRO) market?
Leading companies operating in the Contract Research Organization (CRO) market are IQVIA, Labcorp Drug Development, Parexel International, Syneos Healthand Other prominent players.
Which region holds the largest market share?
North America region hold largest market share in Contract Research Organization (CRO) market
More Related Reports:
Sleep Apps Market

